Chymotrypsin Digestion for Proteomic Analysis: Sigma Protocol Guide

<!DOCTYPE html>
Chymotrypsin digestion is a critical step in proteomic analysis, enabling the breakdown of proteins into peptides for further study. This process is widely used in research to identify and quantify proteins, understand their functions, and explore their roles in various biological processes. The Sigma Protocol provides a standardized and efficient method for chymotrypsin digestion, ensuring reliable and reproducible results. Whether you're a seasoned researcher or just starting in proteomics, this guide will walk you through the process step-by-step, optimizing your workflow for success. Proteomic analysis, protein digestion, chymotrypsin protocol
Understanding Chymotrypsin Digestion in Proteomics
Chymotrypsin is a proteolytic enzyme that cleaves peptide bonds specifically at the carboxyl side of aromatic amino acids like phenylalanine, tryptophan, and tyrosine. This specificity makes it an ideal tool for generating predictable peptide fragments from complex protein mixtures. In proteomic analysis, these fragments are then analyzed using techniques like mass spectrometry (MS) to identify and quantify proteins. Proteolytic enzyme, peptide fragments, mass spectrometry
Materials and Reagents for Chymotrypsin Digestion

Before starting the digestion process, ensure you have the following materials and reagents:
- Chymotrypsin (Sigma-Aldrich)
- Protein sample (e.g., cell lysate, tissue extract)
- Digestion buffer (e.g., 50 mM ammonium bicarbonate, pH 8.0)
- Reducing agent (e.g., dithiothreitol, DTT)
- Alkylating agent (e.g., iodoacetamide, IAA)
- Centrifugal filters (e.g., 10 kDa cutoff)
- Microcentrifuge tubes
Chymotrypsin digestion materials, protein sample preparation, digestion buffer
Step-by-Step Chymotrypsin Digestion Protocol
Step 1: Protein Denaturation and Reduction
1. Dissolve your protein sample in digestion buffer.
2. Add a reducing agent (e.g., 5 mM DTT) and incubate at 56°C for 30 minutes.
3. Cool the sample to room temperature.
📌 Note: Ensure complete denaturation for efficient digestion.
Protein denaturation, reduction, DTT incubation
Step 2: Alkylation of Cysteine Residues
1. Add an alkylating agent (e.g., 10 mM IAA) to the sample.
2. Incubate in the dark at room temperature for 30 minutes.
3. Quench the reaction with an additional 5 mM DTT.
Cysteine alkylation, iodoacetamide, reaction quenching
Step 3: Chymotrypsin Digestion
1. Add chymotrypsin at an enzyme-to-protein ratio of 1:50 (w/w).
2. Incubate at 37°C for 4–18 hours, depending on the sample complexity.
3. Stop the digestion by adding formic acid to a final concentration of 1%.
📌 Note: Longer incubation times may improve peptide coverage but risk over-digestion.
Chymotrypsin enzyme, digestion incubation, formic acid quenching
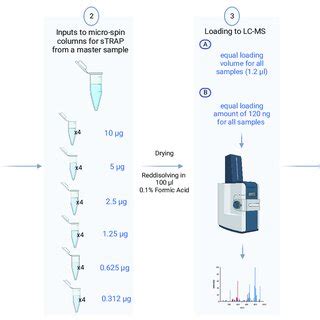
Step 4: Peptide Desalting and Concentration
1. Transfer the digested sample to a centrifugal filter.
2. Centrifuge to remove high molecular weight components.
3. Wash the peptides with 0.1% formic acid and collect the flow-through.
4. Concentrate the peptides using a vacuum centrifuge or speed vacuum.
Peptide desalting, centrifugal filter, peptide concentration
Optimizing Chymotrypsin Digestion for Better Results

To maximize the efficiency and reproducibility of your digestion, consider the following tips:
- Use high-quality chymotrypsin and fresh reagents.
- Optimize the enzyme-to-protein ratio based on your sample.
- Monitor digestion progress using a small aliquot and MS analysis.
- Store peptides at -20°C until ready for analysis.
Digestion optimization, enzyme-to-protein ratio, peptide storage
Checklist for Successful Chymotrypsin Digestion
- Prepare all reagents and materials beforehand.
- Ensure proper denaturation and reduction of proteins.
- Monitor digestion time to avoid over-digestion.
- Desalt and concentrate peptides for optimal MS analysis.
Digestion checklist, protein preparation, peptide analysis
Chymotrypsin digestion is a powerful tool in proteomic analysis, and following the Sigma Protocol ensures consistent and high-quality results. By carefully preparing your samples, optimizing digestion conditions, and handling peptides with care, you can unlock valuable insights into protein structure and function. Whether you're exploring disease mechanisms or studying biological pathways, this protocol will streamline your workflow and enhance your research outcomes. Proteomic research, protein structure, biological pathways
What is the optimal enzyme-to-protein ratio for chymotrypsin digestion?
+A common starting point is a 1:50 (w/w) ratio of chymotrypsin to protein, but this may vary depending on the sample complexity and protein concentration.
How long should chymotrypsin digestion be performed?
+Digestion times range from 4 to 18 hours, with longer times generally improving peptide coverage but increasing the risk of over-digestion.
Can chymotrypsin digestion be used for all types of proteins?
+While chymotrypsin is effective for most proteins, its specificity may limit its use for proteins lacking aromatic amino acids. In such cases, combining it with other enzymes like trypsin can improve coverage.