Trialkyl Sulfonium Ion: Structure, Properties, and Applications
Trialkyl sulfonium ions are versatile chemical species with a wide range of applications in organic synthesis, catalysis, and materials science. Their unique structure, characterized by a positively charged sulfur atom bonded to three alkyl groups, makes them highly reactive and useful in various industrial and research settings. This blog explores the structure, properties, and applications of trialkyl sulfonium ions, providing valuable insights for both informational and commercial audiences.
Structure of Trialkyl Sulfonium Ions
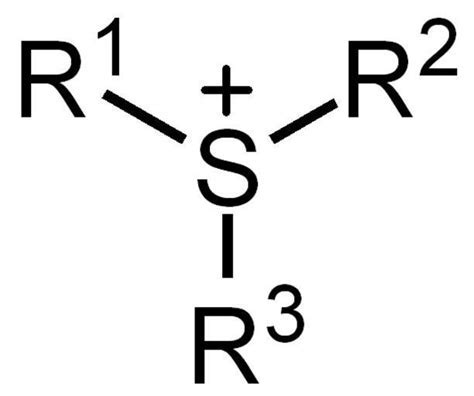
The trialkyl sulfonium ion consists of a central sulfur atom with a positive charge, surrounded by three alkyl groups ®. The general formula is R3S+. This cationic structure is stabilized by the electron-donating nature of the alkyl groups, making it a reactive intermediate in chemical reactions. The flexibility of the alkyl chains allows for diverse functionalization, tailoring the ion for specific applications.
Properties of Trialkyl Sulfonium Ions
Trialkyl sulfonium ions exhibit several key properties that make them valuable in chemistry:
- High Reactivity: The positive charge on sulfur makes it an excellent electrophile, facilitating reactions with nucleophiles.
- Thermal Stability: These ions are stable under moderate conditions, enabling their use in controlled reactions.
- Solubility: Depending on the alkyl groups, they can be soluble in organic solvents, enhancing their utility in synthetic processes.
📌 Note: The reactivity of trialkyl sulfonium ions can be influenced by the length and nature of the alkyl chains.
Applications of Trialkyl Sulfonium Ions
Organic Synthesis
Trialkyl sulfonium ions are widely used as alkylating agents in organic synthesis. They participate in reactions like alkylation, cyclization, and rearrangements, enabling the creation of complex molecules. Their ability to transfer alkyl groups efficiently makes them indispensable in pharmaceutical and fine chemical production.
Photopolymerization
In the field of materials science, trialkyl sulfonium salts act as photoinitiators in polymerization reactions. When exposed to light, they generate free radicals that initiate the curing of resins, making them essential in the production of coatings, adhesives, and 3D printing materials.
Catalysis
These ions also serve as catalysts in various chemical transformations. For example, they are used in asymmetric synthesis to create chiral compounds, which are critical in the pharmaceutical industry. Their catalytic activity can be fine-tuned by modifying the alkyl groups.
| Sulfonium Salt | Application |
|---|---|
| Trimethylsulfonium iodide | Alkylation reactions |
| Triphenylsulfonium hexafluorophosphate | Photopolymerization |
| Triethylsulfonium triflate | Catalysis in organic synthesis |
Checklist for Using Trialkyl Sulfonium Ions
- Determine the desired alkyl group for specific reactivity.
- Choose the appropriate counterion for solubility and stability.
- Optimize reaction conditions for thermal stability.
- Ensure proper safety measures due to their reactive nature.
Trialkyl sulfonium ions are indispensable in modern chemistry, offering unique structural and reactive properties for a variety of applications. Whether in organic synthesis, materials science, or catalysis, their versatility makes them a valuable tool for researchers and industries alike. Understanding their structure and properties is key to harnessing their full potential. (trialkyl sulfonium ion applications, organic synthesis, photopolymerization, catalysis)
What is the general structure of a trialkyl sulfonium ion?
+
The general structure is R3S+, where R represents alkyl groups bonded to a positively charged sulfur atom.
How are trialkyl sulfonium ions used in photopolymerization?
+
They act as photoinitiators, generating free radicals under light exposure to start polymerization reactions in materials like resins and coatings.
What factors influence the reactivity of trialkyl sulfonium ions?
+
The length and nature of the alkyl groups, as well as the counterion, play a significant role in determining their reactivity.